
Copper, Zinc, or Steel Cent
If you think you may have found a transitional cent, such as a 1943 or 1983 Copper cent, there are a few tests you can do to make sure before you submit it to a third party grading company.
First of all, you need to have a scale accurate to at least 1/10th of a gram.
You will need to know how much a coin of a certain metal is supposed to weigh, for example, a Copper planchet should weigh 3.11 grams, a Zinc planchet should weigh 2.5 grams and Steel cent should weigh 2.7 grams. The weight of each can vary a little. The tolerance for the Copper cents is 0.13 grams and 0.10 grams for the Copper plated Zinc cents.
Next we can check for a Steel planchet using a common magnet. If the cent is magnetic, it is most likely Steel. Keep in mind, there are many Copper plated Steel 1943 cents in existance today, some made as novelties and some as counterfeits.
Visually inspect the coin for any alterations or anything that just doesn't look right. Remember that a Copper plated Zinc cent will look just like a 95% Copper cent on the surface, unless it has started to break down with what is commonly called "Zinc Rot". Sometimes areas of Zinc will be exposed letting you see through the Copper plating to the Zinc core, this is a sure way to know that a cent is Copper plated Zinc.
Lets take a suspect coin and see what it turns out to be.
Here, we have a 1983-D Lincoln cent that weighs 2.91 grams. This is too heavy to be a Zinc cent and is just outside of tolerance for a Copper cent.

Visually, this coin looks like Copper, there are no signs of Zinc rot or any other Zinc showing through the surface.
This is where some collectors would be contacting a third party grading service to get it verified as being a Copper/Bronze cent, but there are a couple of more tests we should perform before spending money getting it graded and encapsulated. One test is called a "Specific Gravity" test.
Specific Gravity Test
Density = Mass ÷ Volume
To do this test at home, you will need a scale, accurate to within 1/100th of a gram, a small container of water, and a paper clip. You will also need general knowledge of math or have a calculator handy.
First, we will need to take note of the coin's weight. Write this down as we will need that number for our math later on.
You will need to bend the paper clip so that you will be able to suspend the coin in the container of water.
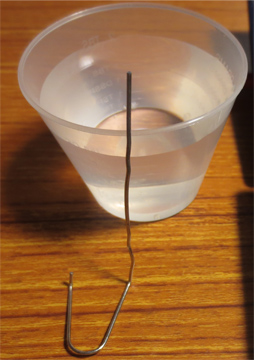
Next, take the container of water and place it on the scale while holding the paper clip in the water, be careful not to touch the bottom or the sides of the conatiner with the paper clip. Do this as the tare weight or in other words the scale must read 0.00g with the container and water on it.

Now it's time to put the cent on the paper clip and suspend it in the water. Take note of the weight when you do this.

Take take the first number, the coin's weight, and divide that by the second number that we got when we suspended the coin in the water. The result will give us the specific gravity of the coin.
The math for our coin is 2.91 / 0.39 = 7.46.
For this number to mean anything to us, we need to know what the specific gravity of the different metals are. Copper has a specific gravity of 8.8 - 8.95 and Zinc has a specific gravity of 6.9 - 7.2.
US cents are not pure Zinc, but Copper plated Zinc (97.5% Zn, 2.5% Cu), and pre-1982 are not pure Copper, but an alloy consisting of 95% Copper and 5% Zinc. The density of a 97.5% Zinc cent is 7.2 g/mL, the density of a 95% Copper cent is 8.8 g/mL and Steel has a specific gravity of 7.7 g/mL - 7.8 g/mL.

If we check some other cents from various years, we see that the volume is almost always 0.370 cubic centimeters. Our coin is .390 cubic centimeters which tells us that the volume of our coin is greater than a normal cent.
We know the volume is greater with our coin than with the others we checked, so it must be thicker, but so little it is not easily discernible with the naked eye. It could be a thick planchet error, but that wouldn't explain the specific gravity results not matching that of a Copper plated Zinc cent.
The specific gravity of our coin is 7.46, which does not match a Copper plated Zinc planchet or a Copper alloy planchet.
With the added weight of our test coin, and the odd results from our specific gravity test, it's time to send it to a third party grading (TPG) company for further testing.
For our TPG we will use NGC (Numismatic Guaranty Corporation) located in Sarasota, Florida. NGC used a variety of tools and tests including, visual, weighing, spectro analysis, specific gravity, et cetera.
After testing, it was determined that this coin was minted on a heavily Copper plated Zinc planchet, a mint error.
The added Copper plating (over 50% of the total planchet composition) accounts for the specific gravity not matching that of a common Copper plated Zinc planchet. It also explains why this coin's volume is greater along with the added weight.

Side Notes
The density of a 97.5% Zinc cent is 7.2 g/mL
The density of a 95% Copper cent is 8.8 g/mL
The density of a Steel cent is 7.7 g/mL.
The diameter of a normal Lincoln cent is 19.05mm.
The Thickness of a normal Lincoln cent is 1.55mm.
A Copper planchet weighs 3.11 grams + - 0.13g
A Zinc planchet weighs 2.5 grams + - 0.10g
A Steel planchet weighs 2.7 grams
Previous Page